There are some important horological anniversaries in 2019, such as 50 years of the Zenith El Primero, 50 years of the TAG Heuer Monaco and 125 years of the Omega calibre. But the world of science celebrates an altogether more epoch-making anniversary that has had a major impact on scientific research, shaped the fields of chemistry and physics and even influenced the watch industry.
The periodic table of the elements was devised by Russian scientist Dmitri Mendeleev in 1869. But the list of elements had first been penned much earlier by French chemist Antoine-Laurent de Lavoisier, who also recognized and named oxygen and hydrogen and, more significantly for the watch industry, predicted the existence of silicon back in 1787. But it was Mendeleev’s table, with its symmetries and orderly classification of the elements (an element being a substance that cannot be broken down by chemical reactions into other substances), that helped to improve our understanding of chemistry and to push the boundaries of research.
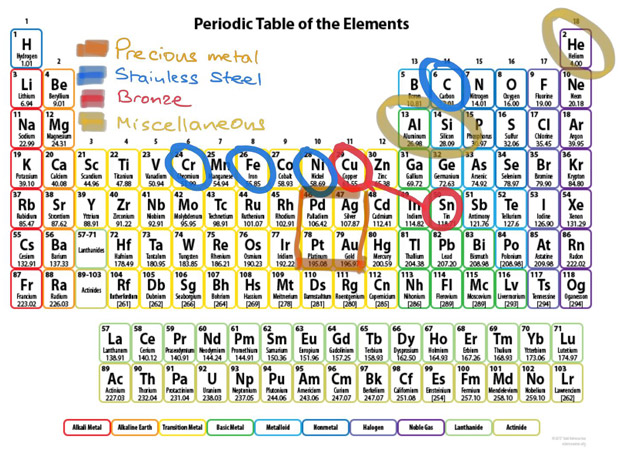
Some of the elements in the periodic table have been staples in the production of watch cases for centuries. In the table of so-called transition metals within the periodic table we find the precious metals of gold (Au) and silver (Ag), as well as the rarer platinum (Pt) and palladium (Pd), which form their own little square in the table. To the left of palladium we find rhodium (Rh) and ruthenium (Ru), which are today used for electroplated movement coatings. Although the resulting coatings have an aesthetically appealing colour and finish, they are primarily used to protect against oxidation of the movement plates and both ruthenium and rhodium are actually more expensive per kilogramme than platinum.
At the top of the transition metals list we also find elements like iron (Fe) which, in combination with its next door neighbours but one on both sides, nickel (Ni) and Chromium (Cr), plus carbon (C) (from the non-metals part of the periodic table), gives us the stainless steel used in the majority of watch cases. In between Fe and Ni we find cobalt (Co), which made its debut in watch cases much more recently. And what would neo-vintage enthusiasts and fans of true diver’s watch have to enthuse about were it not for that wonderful alloy of copper (Cu) and tin (Sn) and its unpredictable patina?
The further left you go along the top row of the transition metals table, the lighter the elements get. Titanium is one of the lightest and has proved itself in many watches, especially when the case diameters start to get larger. But it is not the lightest metal, with scandium to the left of it in the table having a lower atomic weight. I remember seeing a mountain bike offered with a scandium frame in the early 2000s but I have never seen much mention of the metal since. I wonder why watchmakers have never taken to it?
Apart from magnesium in the alkaline earths, there is little among the alkali metals, alkaline earths, lanthanides and actinides in the left half of the table to interest watch brands. And helium (He) at the very top right of the table all on its own as an element lighter than air is the bane of watch brands who produce professional-grade diver’s watches for saturation diving, requiring special valves to release the molecules of this lighter-than-air element from inside the watch.
There are a couple of elements to the right of the transition metals, however, that have only recently become interesting for watch brands. Aluminium, as a metallic metalloid, has been used in watch cases before, but is particularly impressive in the lightest form currently available - called Aeronith - used on some of the latest Zenith models.
Much more important is silicon, which is found among the non-metallic metalloids. Its anti-magnetic properties make it particularly interesting for use as balance springs and escapement components. Since silicon is “grown” rather than machined, it can be produced in any shape. Zenith has recently taken this to a new extreme with its monobloc oscillator. It is a highly complex component but it is made out of one single piece of silicon and replaces some 30 individual components in a conventional regulating organ.
Who knows what other surprises the periodic table may yet reveal for the watch industry. One man who has the answers is Guy Semon, head of the TAG Heuer Institute, which heads up research and development for the brand. Among the high-tech machinery in the institute is a custom-built wall installation of the periodic table with all the information about each element. Samples of the elements themselves will soon be added, too, “even the radioactive ones,” adds Mr Semon with pride. He promises that the periodic table still has a lot in store for the watch industry, and sooner rather than later. Here’s to the next 150 years!




